 |

|
Kansas Geological Survey Open-file Report 2003-33 |
Carbon Dioxide Solubility in Water
The solubility of CO2 in water is a function of temperature, pressure and salinity. The amount of CO2 that can be dissolved in saline brine can be estimated by combining experimental work. The solubility of CO2 in fresh water increases with increasing pressure, decreasing temperature and can be estimated from the experimental work (Crawford and others, 1963; Holm, 1963, Jarrell and others, 2002). A series of solubility curves were used to generate a relational database table of solubility's at different pressure and temperature combinations (Graph A). The solubility's of fresh water in this table are adjusted according to the salinity of the brine (independent of pressure and temperature; Graph B) using percent solubility retained as a function of salinity (Jarrell and others 2002; Chang and others, 1996; Johnson and others, 1952; Martin, 1951). These two figures show the effect of temperature, pressure and salinity on CO2 solubility in water.
CO2 Solubility in Water |
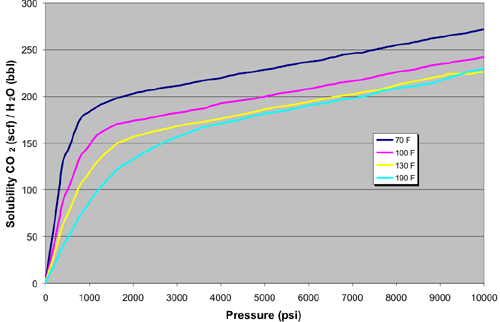 |
Graph A |
CO2 Solubility in Brine |
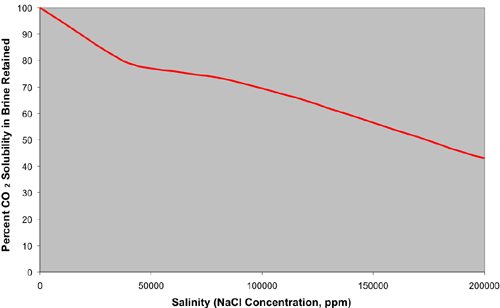 |
Graph B |
-
Chang, Y.B., Coats, B.K., and Nolen, J.S., 1996, A Compositional Model for CO2 Floods Including CO2 Solubility in Water, SPE 35164 presented at the 1996 Permian Basin Oil and Gas Recovery Conference, Midland, Texas, 27-29 March.
Crawford, H.R., Neill, G. H., Bucy, B. J., Crawford, P. B., 1963, Carbon DioxideA Multipurpose Additive for Effective Well Stimulation, Journal of Petroleum Technology, (March 1963) 237.
Holm, L.W., 1963, CO2 Slug and Carbonated Water Oil Recovery Processes, Producers Monthly (September) 6.
Jarrell, P. M., C. E. Fox, M. H. Stein, S. L. Webb, 2002, Practical Aspects of CO2 Flooding, SPE Monograph 22, 220p.
Johnson, W.E., Macfarlane, R.M., and Breston, J.N., 1952, Changes in Physical Properties of Bradford Crude Oil When Contacted with CO2 and Carbonated Water, Producers Monthly (November) 16.
Martin, J.W., 1951, Additional Oil Production Through Flooding with Carbonated Water, Producers Monthly (July) 18.
|
|
Last updated June 2003
http://www.kgs.ku.edu/PRS/publication//2003/2003-33/P1-05.html