Prev Page--Recharge, Discharge || Next Page--Formations
Ground Water, continued
Utilization of Ground Water
Ground water in Lane County is used chiefly for domestic and stock purposes and for public supplies. Ground water used by the Santa Fe Railway has been obtained from the City of Dighton for approximately the last 20 years. A pumping station on the Missouri Pacific Railway in northeast Lane County was abandoned in 1948 as steam locomotives were replaced by diesel. No water is used for industrial purposes as Lane County has no industrial plants. Records of 303 wells in the area are listed in Table 8; the principal uses of water are described below.
Domestic and Stock Supplies
Practically all the domestic and stock supplies in the rural areas and domestic supplies in small towns that have no public water supplies are obtained from wells. One spring is being used as a domestic supply and springs or ponds supplement well water for stock supplies. In Lane County the ground water generally is satisfactory for domestic purposes, although usually moderately hard. Some water with high fluoride content may be injurious to the teeth of children.
Public Supplies
Dighton, the county seat and the only incorporated city in Lane County, has the only public water supply. Homes in smaller communities are supplied from private wells. Dighton (population 1,037 in 1946) is supplied by three drilled wells obtaining water from the Ogallala formation. One well, 18-28-18ccb, is at the power plant and the other two are on the west side of town (18-29-13ddc in the city park and 18-29-13ddb just north of the park). Well 18-29-13ddb is 73 feet deep with a static water level of 52 feet. Well 18-29-13ddc is 87 feet deep and the static water level is 51 feet; well 18-28-18ccb is reported to be about 100 feet deep with a water level of about 53 feet. The wells are cased with 18-inch steel casing and are equipped with electrically driven deep-well turbine pumps. The water from these wells is pumped directly into the city mains, the excess going into an elevated tank. The daily average consumption of water in Dighton is not known, and figures concerning the yields and drawdowns of the wells are not obtainable. An analysis of a sample of water from well 18-29-13ddc is given in Table 5. The water, although moderately hard, is not treated.
Irrigation Supplies
A relatively small amount of water has been pumped for irrigation in Lane County. Prior to 1948 there were only three shallow irrigation wells, these having small yields and irrigating only small areas. In 1948 none of these was in use. Considerable test drilling was done in 1948 and five upland irrigation wells were drilled. However, only two of these, well 16-29-28dab and well 17-28-16dbb, were completed and had pumps installed during the growing season. The acreage irrigated in 1949 has been estimated by Silas Stone, Soil Conservationist, as less than 500 acres. The amount of water used cannot be estimated accurately. A few domestic wells equipped with windmills are sometimes used to irrigate small garden plots, but the amount of water used for this purpose is negligible.
The yields of three irrigation wells were determined by pumping tests made by the Federal Geological Survey. Yields determined by use of the Collins flow meter were 550, 1,040, and 790 gallons a minute. One new well not tested had an estimated yield of about 1,200 gallons a minute. Drawdowns measured by the wetted-tape method during the pumping tests ranged from 36 feet to 12 feet. Results of the pumping tests are given in Table 4. Analyses of two samples of water taken from irrigation wells show that it is of excellent quality for this use.
For data concerning cost of pumping water for irrigation and construction of irrigation plants, the reader is referred to reports prepared by the Kansas State Board of Agriculture (Davison, 1939; McCall and Davison, 1939). A report by Rohwer and Lewis (1940) published by the U. S. Department of Agriculture presents a good discussion of small irrigation pumping plants.
Possibilities of Further Development of Irrigation Supplies
The future development of irrigation in Lane County will be limited by geologic and hydrologic factors. The amount of water available for irrigation depends upon the saturated thickness of water-bearing materials and their permeability. The saturated thickness of Pliocene and Pleistocene deposits in Lane County is shown in Figure 9. The contours showing saturated thickness were prepared by superimposing the water-table contour map (Pl. 1) on the map showing the configuration of the bedrock surface below the Ogallala formation (Fig. 5). Thickness of saturated materials is found by subtracting bedrock altitudes from water-table altitudes at points of intersection. Contour lines are then drawn through points of equal thickness.
Fig. 9--Map showing saturated thickness of the Tertiary and Quaternary deposits in Lane County.
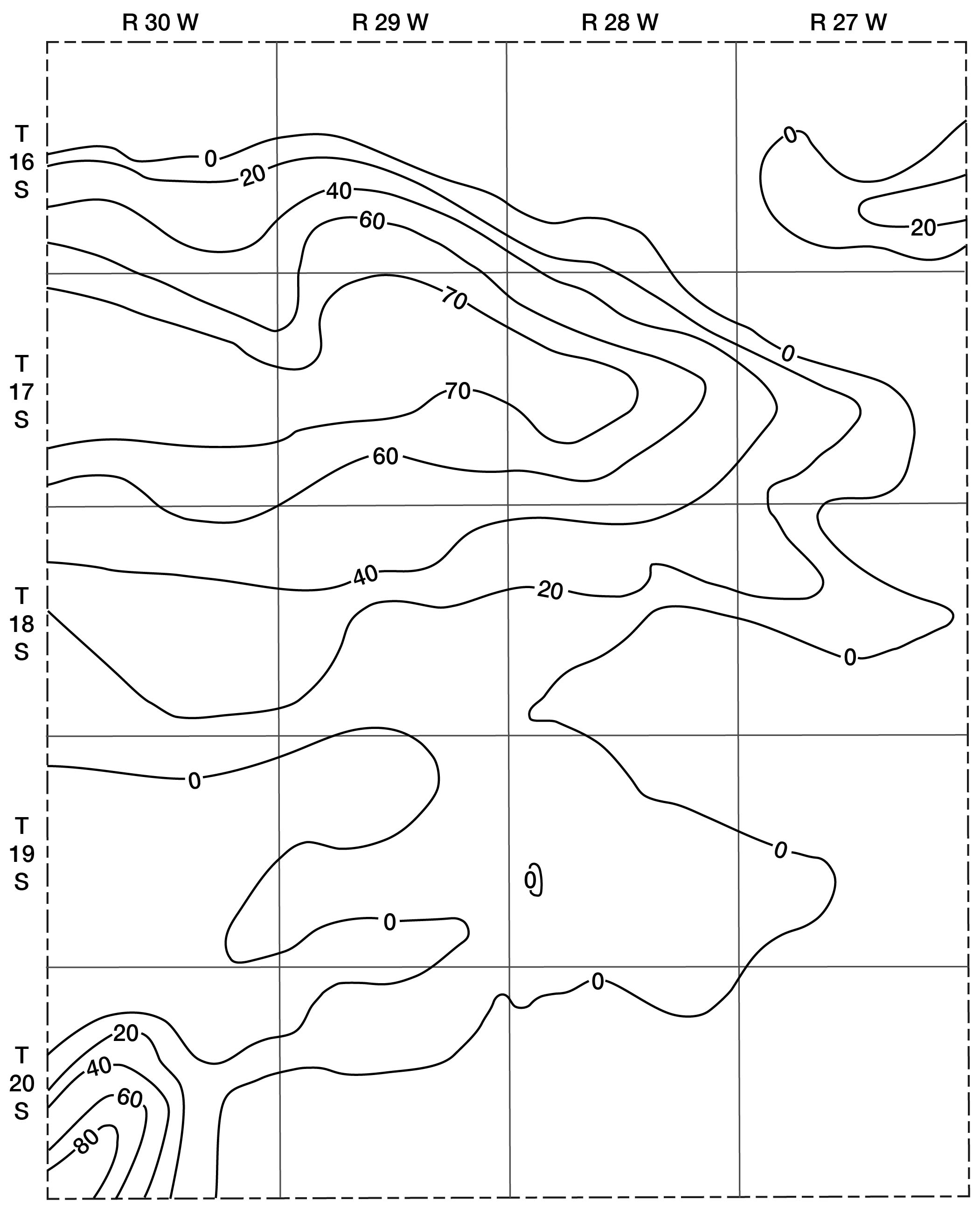
The contours indicate that the saturated water-bearing beds are thin throughout most of Lane County, particularly in the southern half. In many areas where the Cretaceous bedrock is at or near the surface, the Pliocene and Pleistocene deposits are above the water table. In these areas it is difficult to obtain sufficient water for domestic and stock use.
The greatest thickness of saturated Pliocene and Pleistocene materials in Lane County is in the basin area in the southwest corner where the Meade (?) formation contains more than 80 feet of saturated material. Unfortunately, however, the permeability (dependent upon the size and assortment of grains) is not high. Although the amount of water contained in the deposits is large, the material yields water so slowly that wells having large capacities cannot be developed.
An area situated largely in T. 17 S. extending from Scott County eastward for about 16 miles has a saturated thickness of 60 to 70 feet. The permeability of most water-bearing materials in the Ogallala of Lane County is rather low, but large amounts of water can be obtained where materials having high permeabilities are penetrated by wells. Test drilling by the State Geological Survey has shown that in many places the water-bearing beds are too tightly cemented or are composed of such fine materials that wells having large capacities cannot be obtained.
In some of the stream valleys the depths to water are not great and where the alluvium is underlain by the Ogallala formation, it may be possible to obtain supplies large enough for irrigation. However, the narrow creek valleys provide very little land suitable for irrigation.
Chemical Character of Ground Water
The chemical character of ground water in Lane County is shown by analyses of water from 31 wells representing the principal water-bearing formations (Table 5). An analysis of a sample from one of the wells of the City of Dighton is included. Figure 10 shows graphically the chemical character of typical water from the Ogallala, Dakota, and Meade (?) formations and from the alluvium. The samples were analyzed by Howard A. Stoltenberg, chemist, in the Water and Sewage Laboratory of the Kansas State Board of Health at Lawrence. The analyses show only the dissolved mineral content of the waters and do not indicate sanitary condition. General statements on the quality of the ground waters in the county are given below and the quality of waters in the different geologic formations is given in the section on geologic formations and their water-bearing properties.
Fig. 10--Analyses of water from the principal water-bearing formations in Lane County.
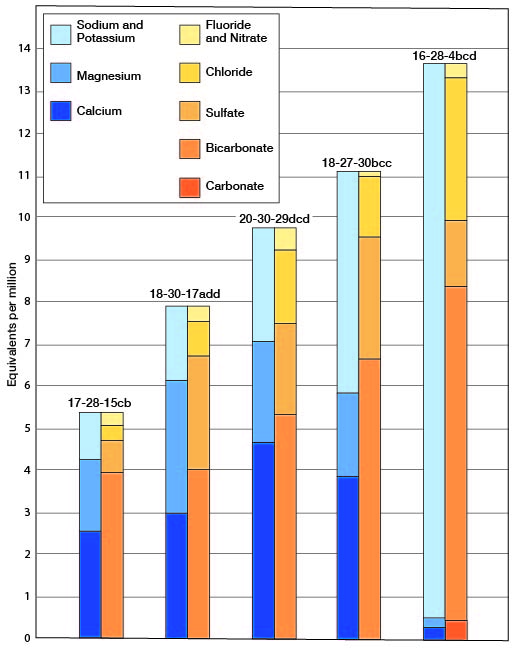
Table 5--Analyses of water front typical wells in Lane County. Analyzed by H. A. Stoltenberg. Dissolved constituents given in parts per milliona, and in equivalents per millionb (in italics).
| Well number | Depth (feet) |
Geologic source |
Date of collection (1948) |
Temp. °F |
Dissolved solids |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium and Potassium (Na+K) |
Bicar- bonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (Fl) |
Nitrate (NO3) |
Hardness as CaCO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Car- bonate |
Noncar- bonate |
||||||||||||||||
| T. 16 S., R. 27 W. | ||||||||||||||||||
| 16-27-13ddd | 80.0 | Ogallala | Sept. 13 | 57 | 270 | 18 | 0.15 | 51 2.54 |
15 1.23 |
22 0.98 |
204 3.34 |
22 0.46 |
21 0.59 |
0.7 0.04 |
20 0.32 |
188 | 167 | 21 |
| T. 16 S., R. 28 W. | ||||||||||||||||||
| 16-28-4bcd | 673c | Dakota | Sept. 14 | 65 | 772 | 7.2 | 1.7 | 4.2 0.24 |
2.8 0.23 |
303 13.17 |
483d 7.92 |
77 1.60 |
120 3.38 |
6 0.32 |
1.1 0.02 |
24 | 24 | 0 |
| 16-28-32caa | 103.0 | Ogallala | Sept. 15 | 496 | 57 | 2.0 | 76 3.79 |
33 2.71 |
31 1.36 |
221 3.62 |
107 2.22 |
47 1.32 |
3.2 0.17 |
33 0.53 |
325 | 181 | 144 | |
| T. 16 S., R. 29 W. | ||||||||||||||||||
| 16-29-11ccc | 749c | Dakota | Sept. 15 | 64 | 932 | 9.2 | 0.98 | 5.2 0.26 |
3 0.25 |
378 16.45 |
734e 12.04 |
39 0.81 |
114 3.21 |
8 0.42 |
0.7 0.01 |
26 | 26 | 0 |
| 16-29-28dab | 170.0 | Ogallala | Sept. 15 | 58 | 332 | 43 | .0 | 52 2.59 |
21 1.73 |
27 1.17 |
232 3.80 |
44 0.92 |
16 0.45 |
2.1 0.11 |
13 0.21 |
216 | 190 | 26 |
| T. 16 S., R. 30 W. | ||||||||||||||||||
| 16-30-13add | 115.0 | Ogallala | Sept. 15 | 59 | 333 | 44 | 1.2 | 55 2.74 |
20 1.64 |
24 1.05 |
227 3.72 |
45 0.94 |
16 0.45 |
1.5 0.08 |
15 0.24 |
219 | 186 | 33 |
| 16-30-21bcb | 147.5 | Ogallala | Sept. 15 | 60 | 311 | 44 | .09 | 52 2.59 |
22 1.81 |
17 0.72 |
214 3.51 |
39 0.81 |
18 0.51 |
2 0.10 |
12 0.19 |
220 | 276 | 44 |
| 16-30-22aba | 36.5 | Ogallala | Sept. 15 | 58 | 339 | 43 | .08 | 57 2.84 |
20 1.64 |
25 1.10 |
234 3.84 |
45 0.94 |
16 0.45 |
1.7 0.09 |
16 0.26 |
224 | 192 | 32 |
| T. 17 S., R. 27 W. | ||||||||||||||||||
| 17-27-16ccc | 30.0 | Ogallala | Sept. 15 | 57 | 386 | 45 | .06 | 84 4.19 |
21 1.73 |
15 0.65 |
283 4.64 |
30 0.62 |
34 0.96 |
1.7 0.09 |
16 0.26 |
206 | 232 | 64 |
| 17-27-35bbb | 110.0 | Ogallala | Sept. 15 | 58 | 322 | 46 | 0.21 | 55 2.74 |
21 1.73 |
20 0.87 |
234 3.84 |
35 0.73 |
16 0.45 |
2.4 0.13 |
12 0.19 |
224 | 192 | 32 |
| T. 17 S., R. 28 W. | ||||||||||||||||||
| 17-28-15cb | 147.0 | Ogallala | Sept. 14 | 58 | 326 | 48 | 0.13 | 51 2.54 |
21 1.73 |
25 1.08 |
239 3.92 |
37 0.77 |
12 0.34 |
2.6 0.14 |
11 0.18 |
214 | 196 | 18 |
| T. 17 S., R. 29 W. | ||||||||||||||||||
| 17-29-31aaa | 44.0 | Ogallala | Sept. 15 | 57 | 393 | 43 | 0.08 | 60 2.99 |
26 2.14 |
32 1.41 |
257 4.21 |
51 1.06 |
24 0.68 |
3 0.16 |
27 0.43 |
256 | 210 | 46 |
| T. 18 S., R. 27 W. | ||||||||||||||||||
| 18-27-30bcc | 20.5 | Alluvium | Sept. 15. | 57 | 654 | 38 | 0.04 | 77 3.84 |
24 1.97 |
121 5.26 |
404 6.82 |
140 2.91 |
51 1.44 |
1.1 0.06 |
2.7 0.04 |
290 | 290 | 0 |
| T. 18 S., R. 28 W. | ||||||||||||||||||
| 18-28-16ccc | 68.0 | Ogallala | Sept. 14 | 58 | 438 | 70 | 0.08 | 55 2.74 |
35 2.88 |
33 1.45 |
261 4.28 |
75 1.56 |
34 0.96 |
3.8 0.20 |
4.4 0.07 |
281 | 214 | 67 |
| T. 18 S., R. 29 W. | ||||||||||||||||||
| 18-29-1dcc | 65.5 | Ogallala | Sept. 14 | 58 | 361 | 59 | 1.2 | 58 2.89 |
23 1.89 |
25 1.10 |
260 4.26 |
35 0.73 |
18 0.51 |
3.8 0.20 |
11 0.18 |
239 | 213 | 26 |
| 18-29-13ddc | 88.5 | Ogallala | Aug. 1f | 460 | 56 | 0.13 | 58 2.89 |
33 2.71 |
27 1.19 |
278 4.56 |
45 0.94 |
31 0.87 |
3.4 0.18 |
15 0.24 |
280 | 228 | 52 | |
| 18-29-20bbb | 63.5 | Ogallala | Sept. 15 | 58 | 333 | 67 | 0.06 | 40 2.00 |
28 2.30 |
26 1.13 |
270 4.43 |
18 0.37 |
10 0.28 |
4.5 0.24 |
6.6 0.11 |
215 | 215 | 0 |
| 18-29-36aaa | 47.0 | Ogallala | Sept. 14 | 58 | 592 | 42 | 0.03 | 106 5.29 |
34 2.79 |
31 1.33 |
277 4.54 |
67 1.39 |
51 1.44 |
0.8 0.04 |
124 2.00 |
404 | 227 | 177 |
| T. 18 3., R. 30 W. | ||||||||||||||||||
| 18-30-17add | 96.5 | Ogallala | Sept. 16 | 60 | 483 | 52 | 0.14 | 60 2.99 |
38 3.12 |
41 1.78 |
244 4.00 |
130 2.70 |
30 0.85 |
4 0.21 |
8 0.13 |
306 | 200 | 106 |
| 18-30-34aba | 93.5 | Ogallala | Sept. 16 | 58 | 362 | 57 | 0.18 | 41 2.05 |
29 2.38 |
34 1.50 |
242 3.97 |
48 1.00 |
21 0.59 |
4.5 0.24 |
8 0.13 |
222 | 198 | 24 |
| T. 19 S., R. 27 W. | ||||||||||||||||||
| 19-27-33dad | 20.4 | Alluvium | Sept. 16 | 61 | 789 | 28 | 0.04 | 190 9.48 |
24 1.97 |
42 1.83 |
433 7.10 |
241 5.01 |
28 0.79 |
0.6 0.03 |
22 0.35 |
572 | 355 | 217 |
| T. 19 S., R. 28 W. | ||||||||||||||||||
| 19 28-35cbb | 72.0 | Ogallala | Sept. 16 | 59 | 412 | 29 | 0.25 | 61 3.04 |
26 2.14 |
45 1.94 |
245 4.02 |
67 1.39 |
53 1.49 |
1.5 0.08 |
8.8 0.14 |
259 | 201 | 58 |
| T. 19 S., R. 29 W. | ||||||||||||||||||
| 19-29-22aad | 73.7 | Ogallala | Sept. 16 | 58 | 380 | 23 | 0.92 | 51 2.54 |
21 1.73 |
54 2.34 |
254 4.17 |
67 1.39 |
30 0.85 |
1.4 0.07 |
8 0.13 |
214 | 208 | 6 |
| T. 19 S., R. 30 W. | ||||||||||||||||||
| 19-30-30add | 1,038c | Dakota | Sept. 16 | 69 | 669 | 8 | 4.2 | 11 0.55 |
5.2 0.43 |
238 10.33 |
331 5.43 |
154 3.20 |
67 1.89 |
5 0.26 |
3.5 0.06 |
49 | 49 | 0 |
| 19-30-35daa | 74.0 | Ogallala | Sept. 16 | 59 | 712 | 27 | 2.5 | 84 4.19 |
42 3.45 |
92 4.02 |
261 4.28 |
150 3.12 |
98 2.76 |
1.3 0.07 |
89 1.43 |
382 | 214 | 168 |
| T. 20 S., R. 27 W. | ||||||||||||||||||
| 20-27-7aab | 748c | Dakota | Sept. 16 | 67 | 1,174 | 8.6 | 3.4 | 4.8 0.24 |
2.6 0.21 |
447 19.45 |
470h 7.71 |
207 4.31 |
248 6.99 |
6 0.32 |
2.6 0.04 |
22 | 22 | 0 |
| 20-27-19bbc | 31.7 | Alluvium | Sept. 16 | 58 | 399 | 18 | 0.08 | 90 4.49 |
14 1.15 |
28 1.20 |
293 4.80 |
45 0.94 |
10 0.28 |
0.6 0.03 |
49 0.79 |
282 | 240 | 42 |
| 20-27-26bba | 34.5 | Alluvium | Sept. 16 | 59 | 449 | 20 | 0.11 | 101 5.04 |
15 1.23 |
32 1.38 |
312 5.12 |
58 1.21 |
16 0.45 |
0.4 0.02 |
53 0.85 |
314 | 256 | 58 |
| 20-27-31cbb | 23.8 | Alluvium | Sept. 16 | 59 | 450 | 19 | 0.06 | 114 5.69 |
11 0.90 |
18 0.79 |
249 4.08 |
55 1.14 |
30 0.85 |
0.4 0.02 |
80 1.29 |
330 | 204 | 126 |
| T. 20 S., R. 28 W. | ||||||||||||||||||
| 20-28-19aaa | 47.5 | Ogallala | Sept. 14 | 57 | 571 | 32 | 0.15 | 127 6.34 |
35 2.88 |
18 0.77 |
454 7.44 |
16 0.33 |
22 0.6 |
0.7 0.04 |
97 1.56 |
461 | 372 | 89 |
| T. 20 S., R. 30 W. | ||||||||||||||||||
| 20-30-29dcd | 30.0 | Meade (?) | Sept. 16 | 56 | 562 | 20 | 3.00 | 93 4.64 |
29 2.38 |
63 2.74 |
324 5.31 |
104 2.16 |
63 1.78 |
0.7 0.04 |
29 0.47 |
351 | 266 | 85 |
| a. One part per million is equivalent to one pound of substance per million pounds of water or 8.33 pounds per million gallons of water. b. An equivalent per million is a unit chemical equivalent weight of solute per million unit weights of solution. Concentration in equivalents per million is calculated by dividing the concentration in parts per million by the chemical combining weight of the substance or ion. c. Depth reported. d. Sample also contains 12 ppm carbonate (.40 equivalents per million). e. Sample also contains 14 ppm carbonate (.47 equivalents per million). f. Sample collected in 1947 (Dighton municipal well). g. Sample also contains 14 ppm carbonate (.47 equivalents per million). h. Sample also contains 16 ppm carbonate (.53 equivalents per million). |
||||||||||||||||||
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water has been adapted in part from publications of the Federal Geological Survey and the State Geological Survey of Kansas.
Dissolved solids--Ground water dissolves some of the rock materials with which it comes in contact. After a natural water has been evaporated, the residue consists of mineral matter and some organic material and some water of crystallization. The kind and quantity of these materials in the water determine its suitability for various uses. Waters containing less than 500 parts per million of dissolved solids generally are satisfactory for domestic use, except for troubles resulting from their hardness and corrosiveness. Waters containing more than 1,000 parts per million dissolved solids generally are not satisfactory for most domestic and industrial uses for they are likely to contain enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respect.
The water from only one of the wells sampled in Lane County contained more than 1,000 parts per million (1,174) dissolved solids. Nine samples contained from 500 to 1,000 and 21 contained less than 500, the smallest concentration being 270 parts per million. The amount of dissolved solids in the samples of ground water collected in Lane County is indicated for each important aquifer in Table 6.
Table 6--Summary of the chemical character of samples of water from typical wells in Lane County.
| Range in parts per million |
Number of samples | |||
|---|---|---|---|---|
| Ogallala formation |
Alluvium | Meade (?) formation |
Dakota formation |
|
| Dissolved solids | ||||
| 0-200 | 0 | 0 | 0 | 0 |
| 201-300 | 1 | 0 | 0 | 0 |
| 301-400 | 13 | 1 | 0 | 0 |
| 401-500 | 4 | 2 | 0 | 0 |
| 501-600 | 2 | 0 | 1 | 0 |
| More than 600 | 1 | 2 | 0 | 4 |
| Total hardness | ||||
| 0-50 | 0 | 0 | 0 | 4 |
| 51-100 | 0 | 0 | 0 | 0 |
| 101-200 | 1 | 0 | 0 | 0 |
| 201-300 | 15 | 2 | 0 | 0 |
| 301-400 | 3 | 2 | 1 | 0 |
| 401-500 | 2 | 0 | 0 | 0 |
| More than 500 | 0 | 1 | 0 | 0 |
| Fluoride | ||||
| Less than 0.5 | 0 | 2 | 0 | 0 |
| 0.5-1.0 | 3 | 2 | 1 | 0 |
| 1.1-1.5 | 4 | 1 | 0 | 0 |
| 1.6-3.0 | 7 | 0 | 0 | 0 |
| 3.1-5.0 | 7 | 0 | 0 | 1 |
| 5.1-8.0 | 0 | 0 | 0 | 3 |
| Iron | ||||
| Less than 0.10 | 8 | 4 | 0 | 0 |
| 0.10-0.20 | 6 | 1 | 0 | 0 |
| 0.21-0.50 | 2 | 0 | 0 | 0 |
| 0.51-1.0 | 1 | 0 | 0 | 1 |
| 1.1-2.0 | 3 | 0 | 0 | 1 |
| 2.1-3.0 | 1 | 0 | 1 | 0 |
| 3.1-5.0 | 0 | 0 | 0 | 2 |
Hardness--The hardness of water is the property that receives the most attention and is commonly recognized by the increasing amount of soap needed to produce a lather and by the curdy precipitate that forms before a permanent lather can be obtained. Calcium and magnesium are the constituents that cause practically all hardness of ordinary waters and are the active agents in the formation of the greater part of the scale formed in steam boilers and in other vessels in which water is heated or evaporated.
In addition to the total hardness, the table of analyses (Table 5) shows carbonate and noncarbonate hardness. Carbonate hardness is that caused by calcium and magnesium bicarbonate and, because it can be almost entirely removed by boiling, it is sometimes called temporary hardness. Noncarbonate hardness, often called permanent hardness, is caused mainly by sulfates or chlorides of calcium and magnesium and cannot be removed by boiling. With reference to use with soap there is no difference between carbonate and noncarbonate hardness. In general, waters with high permanent hardness tend to form harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft and treatment to remove hardness is unnecessary. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but it does increase the consumption of soap and its removal by a softening process may be profitable for laundries or other industries that use large quantities of soap. Treatment for the prevention of scale is necessary for the successful operation of steam boilers using water in the upper part of this range of hardness. Water having a hardness between 200 and 300 parts per million is sometimes treated to soften it to the point where it is suitable for household use. Sometimes cisterns to collect soft rain water are installed. Where municipal water supplies are softened the hardness is usually reduced to 60 to 80 parts per million.
Four analyses of water from the Dakota formation showed a hardness of less than 50 parts per million, and may be considered soft. Most of the waters derived from other formations, however, were quite hard, only 1 having a hardness of less than 200 parts per million; 9 samples had a hardness of more than 300 parts per million and 17 had a hardness of from 200 to 300.
Iron--Next to hardness, iron content is the property of natural waters that usually receives the most attention. If a water contains more than 0.3 part per million of iron, the excess may separate out and settle as a reddish sediment when exposed to the air. Iron, which may be present in sufficient quantity to give a disagreeable taste or to stain cooking utensils, may be removed from most waters by aeration and filtration, but a few waters require additional treatment.
Maximum iron content in water samples collected in Lane County was 4.2 parts per million. One sample contained no iron; 11 others contained less than 0.1 part per million. All but three had less than 3 parts per million.
Fluoride--Although fluoride is usually present only in small quantities in ground water, it is desirable to know the amount of fluoride in waters that are used by children. Fluoride in water has been shown to be associated with the dental defect known as mottled enamel, which may appear on the teeth of children who drink water containing fluoride during the period of formation of the permanent teeth. Dean (1936, pp. 1278-1279) has described the effects of fluoride in drinking water on the teeth of children as follows:
. . . from the continuous use of water containing about 1 part per million, it is probable that the very mildest forms of mottled enamel may develop in about 10 percent of the group. In water containing 1.7 or 1.8 parts per million, the incidence may be expected to rise 40 or 50 percent, although the percentage distribution of severity would be largely of the "very mild" and "mild" types. At 2.5 parts per million an incidence of about 75 to 80 percent might be expected, with possibly 20 to 25 percent of all cases falling into the "moderate" or severer type. A scattering few may show the "moderately severe" type.
At 4 parts per million the incidence is, in general, in the neighborhood of 90 percent, and as a rule, 35 percent or more of the children are classified as "moderate" or worse. In concentration of 6 parts per million or higher an incidence of 100 percent is not unusual. In other words, we are dealing with a low grade chronic fluorine poisoning of children.
A more recent report (Dean, Arnold, and Elvolve, 1942) has indicated that although more than one part per million of fluoride may be detrimental to the teeth of children, small amounts of fluoride are beneficial in helping to prevent tooth decay.
Of 31 samples from Lane County, only 8 contained less than 1 part per million of fluoride, 20 contained from 1 to 5 parts per million and 3 contained more than 5. Water from one well drilled to the Dakota formation had a fluoride content of 8.0 parts per million.
Nitrate--The nitrate content of waters used for drinking has received a great deal of attention in the past few years. This concern is due to the discovery that high nitrate water is associated with cyanosis of infants when the water is used in the preparation of the baby's formula. The variation in nitrate content for different water samples is great and apparently is not related to any geologic formation. Although some nitrates may be derived from nitrate-bearing rocks and minerals in the water-bearing formation, high nitrate concentrations probably are due to direct flow of surface water into the well or to percolation of nitrate-bearing water into the well through the top few feet of the well. Nitrates are very soluble and may be dissolved readily from soils that have high concentrations of nitrate and from barnyard refuse. Dug wells, in most cases poorly sealed, generally allow more contamination by surface seepage than do drilled wells, which are ordinarily deeper and more tightly sealed at the surface.
Water having nitrate concentration greater than about 50 parts per million of nitrate as NO3 should not be used for formula preparation. Although all the water samples collected contained some nitrate, only 5 samples contained more than 50 parts per million. Nitrate content ranged from 0.7 in a deep well to the Dakota formation to 124 parts per million in one shallow dug well in the Ogallala formation.
Water for Irrigation
The suitability of water for irrigation is dependent mainly on the total concentration of dissolved constituents and the percentage of sodium. A large quantity of chloride may make water unfit for irrigation and boron may be present in sufficient amounts to be harmful to plants. The total concentration of dissolved constituents may be expressed in terms of total equivalents per million of anions and cations, in terms of parts per million of dissolved solids, or in terms of electrical conductivity. Electrical conductivity is the measure of the ability of the organic salts in solution to conduct an electrical current, and it is related to the concentration of dissolved solids. Electrical conductivity measurements are not shown in analyses of water from Lane County, but an approximate value can be obtained by multiplying total equivalents per million of anions or cations by 100, or by dividing dissolved solids in parts per million by 0.7 (Wilcox, 1948, pp. 4-5). To find the percentage of sodium the results of the analysis must be reported in equivalents per million. The quantity of sodium in equivalents is divided by the sum of the quantities of calcium, magnesium, sodium, and potassium and the result is expressed as a percentage.
The classification of waters for irrigation use is shown in Table 7 (Wilcox, 1948a, p. 27).
Table 7--Permissible limits for electrical conductivity and percentage sodium of several classes of irrigation water (Wilcox, 1948a, p. 27).
| Classes of water | Electrical conductivity (micromhos at 25° C) |
Percent sodium |
|
|---|---|---|---|
| Rating | Grade | ||
| 1 | Excellent | less than 250 | less than 20 |
| 2 | Good | 250-750 | 20-40 |
| 3 | Permissible | 7,50-2,000 | 40-60 |
| 4 | Doubtful | 2,000-3,000 | 60-80 |
| 5 | Unsuitable | more than 3,000 | more than 80 |
From Table 7 it can be said that in general, waters containing more than 60 percent sodium or waters having electrical conductances of more than 2,000 are unfit for irrigation. In Lane County water from four wells drilled to the Dakota formation was unfit for irrigation.
Sanitary Conditions
The analyses of water given in the table show only the amounts of dissolved mineral matter in the water and do not indicate the sanitary quality of the water. The water in a well containing mineral matter that imparts an objectionable taste or odor may be free from harmful bacteria and safe for drinking. On the other hand, the water in a well although clear and palatable may contain harmful bacteria. An abnormal amount of certain mineral constituents, such as nitrate or chloride, however, may indicate pollution.
Great care should be taken to protect domestic and public water supplies from contamination by organic material. Much of the population of Lane County is dependent upon private water supplies from wells, and drillers and well owners must take precautions in constructing wells to insure safe and wholesome water supplies. The top of the casing should be sealed in such a manner as to prevent surface water from entering the well, and where pump pits are used the top of the casing should extend above the floor of the pit to prevent surface water from draining into the well. In constructing wells equipped with ordinary cylinder pumps it is a good plan to allow the casing to extend several inches above the platform so that the pump base will fit down over the top of the casing, thus effecting a tight seal. If the casing is left flush with the top of the platform opportunity for surface drainage into the well and for possible contamination is afforded. Wells should not be located where barnyards, privies, or cesspools are possible sources of contamination.
Prev Page--Recharge, Discharge || Next Page--Formations
Kansas Geological Survey, Geology
Placed on web Jan. 18, 2008; originally published Sept., 1951.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Lane/05_gw3.html